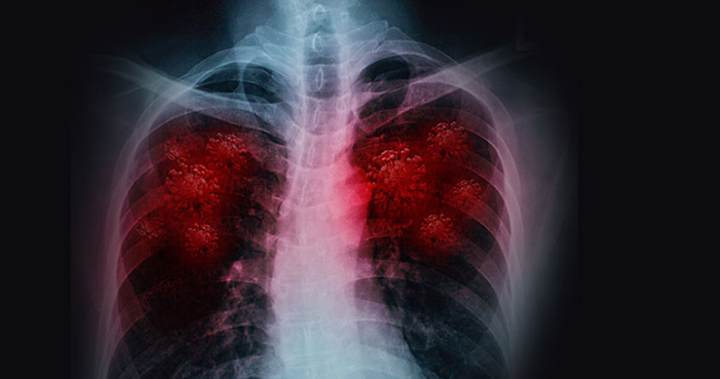
Pulmonem Inc. was founded to demonstrate the effectiveness and safety of PULM-001, a patented and reformulated generic drug, to arrest the development of inflammation caused by COVID-19 and help prevent hospitalization of those who become infected. Pulmonem has established manufacturing partnerships that will allow the Company to quickly bring this treatment to market soon after successful completion of clinical trials.
Pulmonem is also pursuing solutions for the pediatric market, as well as alternative delivery systems, such as nasal sprays and liquids.
Latest News
The Innovation
Pulmonem is developing and testing a therapy to treat COVID-19 with a newly patented oral medication to arrest the development of inflammation caused by COVID-19 infection, preventing the excessive immune reaction that is the most frequent cause of worsening symptoms and complications requiring hospitalization.
COVID-19 has already affected more than 24 million people around the world and severely disrupted the global economy. By neutralizing the aspect of COVID-19 that causes the gravest complications and fatalities, Pulmonem’s medication could allow for a return to “normal life”, while the world awaits a vaccine.
The therapy developed by Pulmonem is unique in that it is a reformulation of a safe and affordable generic anti-inflammatory drug that has been around for decades, used to treat malaria, lupus, HIV and other inflammatory infections. Because the new medication is a reformulation of an existing drug for a new indication, this therapy could be available on a much-accelerated timeline compared to others in development.
Pulmonem has manufacturing partnership agreements in place and is ready to start producing its new medication for the treatment of COVID-19 quickly and in large quantities. Manufacturing is set to take place in New Brunswick and in the U.S. for immediate worldwide distribution upon the successful completion of the trial and regulatory approvals.
Pulmonem’s medication could be ready to be administered to Canadians before the end of 2020.
DAP-CORONA Clinical Trial
Pulmonem will be the first company to investigate the use of PULM-001 as a treatment for COVID-19. The Company will test an early treatment approach to arresting COVID-19 complications among non-hospitalized, confirmed cases during its DAP-CORONA Phase 3 Clinical Trial.
The Phase 3 Clinical Trial is sponsored by the Research Institute of the McGill University Health Centre (RI-MUHC).
DAP-CORONA is a randomized, triple-blind, placebo-controlled, parallel, multicentre trial to evaluate the efficacy and safety of PULM-001 in older adults (70+ years of age) and/or in adult patients (40+ years of age) with at least one high-risk comorbidity, with confirmed COVID-19 infection.
The trial will comprise a total of 2,100 infected patients diagnosed with COVID-19, non-hospitalized at the time of enrolment and meeting all inclusion and no exclusion criteria. They will be randomized to receive either PULM-001 tablets or placebo tablets for 21 days and will be followed up for 30 days after termination of treatment.
The Advantages of Our Solution
- We believe that our therapy will alleviate and may even prevent the pulmonary inflammatory phase of COVID-19, helping prevent the hospitalization of those who become infected.
- The generic drug we are testing has been successfully used as an anti-inflammatory and has been used to treat other infectious diseases, including malaria, lupus, HIV and pneumocystis jirovecii pneumonia.
Our Solution:
- is based on a generic and thus affordable.
- is efficiently absorbed (70% to 80%) via the gastrointestinal tract and is therefore suitable for outpatient settings.
- reaches peak serum concentration in 2 to 6 hours and has a half-life of 20 to 30 hours.
- is well distributed to the fluid of the alveolar spaces in the lung.
- has well-documented metabolic, pharmacokinetic and toxicological profiles.
Management Team

Houfar Sekhavat, MD
Founder and Chairman
Ophthalmologist, serial entrepreneur and innovator. Founder of Triple Hair Inc. (hair growth therapies) and Hexiris Pharma Inc. (treatment of glaucoma).

Jean-Philippe Gravel, MBA
Co-Founder and Chief Operating Officer
Over 15 years of experience in management and business development, mainly with medical, pharmaceutical and natural health product companies. President and CEO of Triple Hair Inc.

Guy Chamberland, Ph.D.
CEO
Over 27 years of experience in management, drug development and regulatory affairs with medical, pharmaceutical and natural health product companies.

Denis Albert, CPA, CA
Co-Founder
Managing partner at Boudreau Albert Savoie & Associates, in charge of operations, strategic direction and client relationships, with over 21 years of experience in accounting, tax planning and business-related matters, including tax and financial restructuring, financing, business sales and acquisitions.

Awdah Arraf, Ph.D.
Co-Founder
Patent and innovation strategy specialist with over 20 years of experience helping tech and pharma start-ups establish and exploit patent portfolios. Engaged in science and technology, she dedicates her skills to organizations with profound products and services that will ultimately contribute to enhancing the lifestyle and wellbeing of individuals globally.

Chanele Dumontier, CPA
Chief Financial Officer
Extensive experience in finance and accounting in leading international companies with a focus on growth strategies, mergers and acquisitions and partnership development.

Satish Asotra, Ph.D., MBA
Chief Manufacturing and Regulatory Officer
Senior executive and strategic leader with a proven track record of driving pharmaceutical development, innovation and process improvement with 25 years of experience, including more than 12 years in topical and dermatological product development, with Avicanna, AHI, Accucaps, Odan Laboratories and Taro Pharmaceuticals.

Georges H. Lavanchy
Vice President, International Development
Entrepreneur with international management experience in various fields for over 30 years. Excellent knowledge of European markets and their business practices. Searching for innovative products to be developed.

Avraham Yacobi, Ph.D., M.Pharm.
Principal Scientific Advisor
Expert consultant with over 35 years of experience in pharmaceutical R&D and regulatory affairs. Previously served as President of R&D at Taro Pharmaceuticals USA for 16 years, and as Senior VP and Officer at Taro Pharmaceutical Industries. Currently President of DOLE Pharma, LLC and Founder and Scientific Advisor to BluPax Pharma.
Looking to Invest?
Interested in investing or participating in the Clinical Trial?
Please complete the form below or email us at info@pulmonem.ca.
Pulmonem Inc.
855, Dieppe Blvd
Dieppe, NB E1A 7R8
CANADA
Media Enquiries
Keelan Green
613-220-2016
green@prospectus.ca